Alcohols
An alcohol is a hydrocarbon with an -OH bonded to it! The same naming rules apply as usual, but the parent chain ending is -ol.
Example

The carbon chain has TWO carbons so we know that there will be eth- as the beginning of the parent chain! And the ending will be -ol, so putting it together, thats ETHANOL!
Let's try a harder example:

The longest parent chain has 3 carbons, making the prefix prop-
There is a methyl side chain coming from the 2nd carbon, and the OH, making it an alcohol, is also on the 2nd carbon!
That makes this 2 methyl 2 propanol
Also, a note to remember: When benzene makes alcohol, put an OH anywhere (attached to any carbon) and it will be called "Phenol".
If you want to get to know alcohol a little bit better, you can read this page!
http://www.talktalk.co.uk/reference/encyclopaedia/hutchinson/m0009891.html
Now, on to Halides!
Group 7 elements (F, Cl, Br, I) can bond to a hydrocarbon chain as well! Naming follows the exact same rules, except that you add the prefix floro- , chloro-, bromo-, and iodo- accordingly
Some examples:
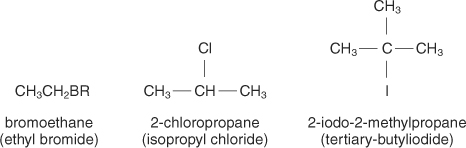
Ignore the one on the left!
So for the middle one, there is a chloro from the second carbon in the propane chain, so it will be called 2 chloro propane!|
For the one on the right, there is an iodine on the second one, and there is also a methyl. This makes it 2 iodo 2 methyl propane!
A last, more complicated example:

No comments:
Post a Comment